While homogeneous catalysis remains predominantly dominated by transition metal complexes, the utilization of main group compounds as redox catalysts for diverse chemical transformations represents a long-sought yet underexplored frontier. This work reports the catalytic reduction of N2O in the presence of HBpin via an unprecedented EII/EIVO (E = Ge, Sn) redox platform, employing carbodiphosphorane ligated germylene or stannylene as the catalyst. The key GeIV and SnIV-oxo intermediates have been isolated and characterized. Furthermore, we extend this redox platform to achieve efficient and chemodivergent reduction of nitroarenes under mild conditions. Notably, through systematic modulation of reaction conditions, four different reduced products including amino, hydroxylamine, azoxy, and hydrazine derivatives can be synthesized from nitroarenes with high yields and selectivity. This work establishes a novel heavy group 14 element-based catalytic manifold and unlocks new opportunities for the application of heavy carbene analogues in redox catalysis.
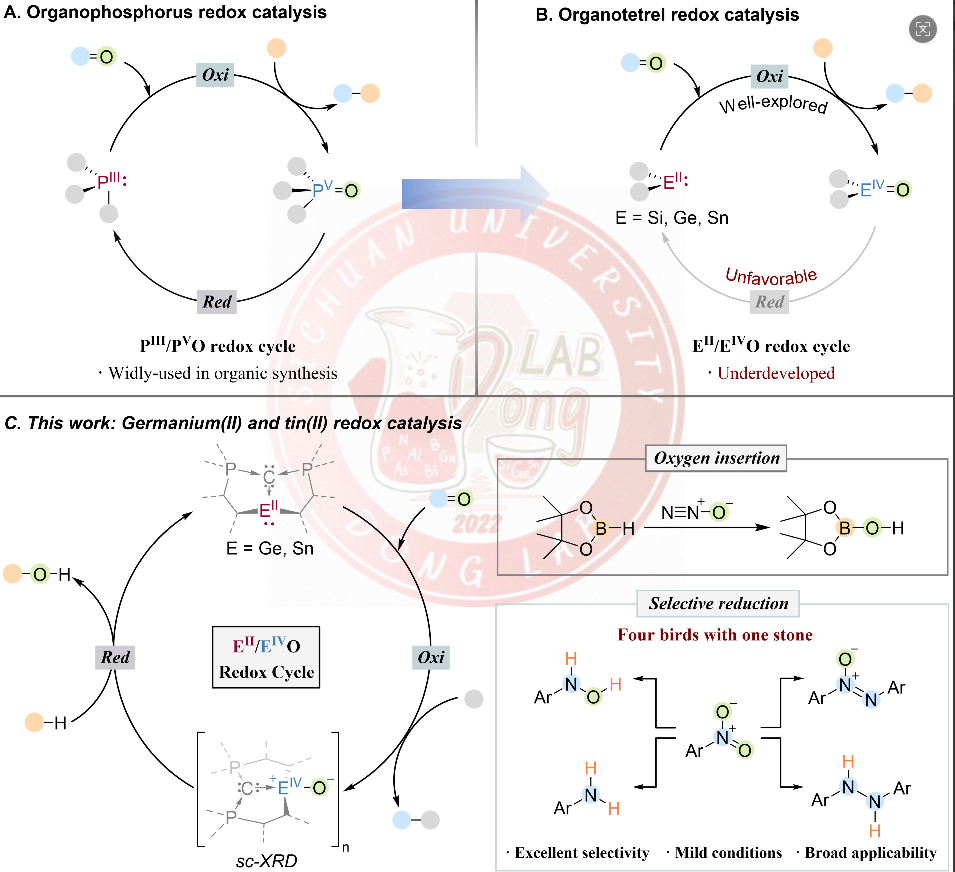
This work was published in Angew. Chem. Int. Ed. entitled “EII/EIVO (E = Ge, Sn) Catalyzed Nitrous Oxide Activation and Chemodivergent Reduction of Nitroarenes” (https://doi.org/10.1002/anie.202515638). Prof. Zhaowen Dong is the corresponding author of the paper, and Ph.D. candidate Zhuchunguang Liu, Master’s student Xiaojian Li and research assistant Zhijun Wang are the co-first authors. This work was financially supported by National Natural Science Foundation of China, the National Key R&D Program of China, and Natural Science Foundation of Sichuan, China and the Fundamental Research Funds for the Central Universities.
